We talked to scientist Robert Dominko, who has been researching batteries for a long time now. The National Institute of Chemistry in Ljubljana has been making, piercing, burning and measuring batteries for decades in a refined manner. It has been adding new compounds in search of ways to chemically store electric energy in a more efficient way and then return it.
As it turns out, their eureka moment has arrived. One of their researches, carried out in cooperation with the Paris-based College de France, ended up in Science magazine, which together with Nature is considered one of the most prestigious science publications in the world. The reason? The international team came up with a way to boost the capacity of a battery, with the same volume, by 50 percent.
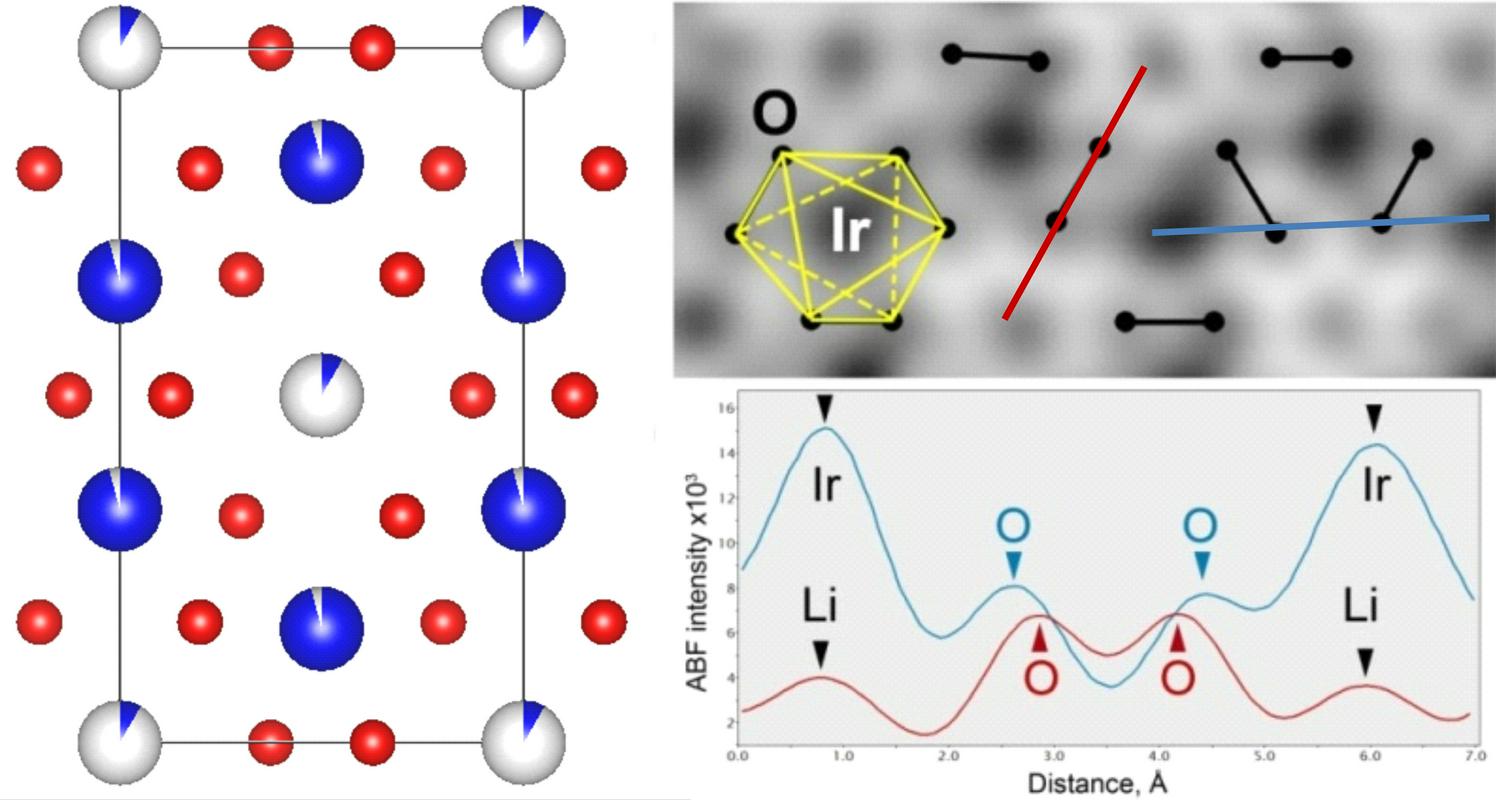
How did they succeed? With the visualization of O-O peroxo-like dimers in cathode materials with high energy density. "If was really that simple, it wouldn't be published in Science magazine," joked Dominko. He has been researching Lithium-ion batteries together with his postdoctoral researcher and Paris colleagues.
Batteries which store electric energy use a metal called Lithium. The group of researchers proved that we can also store electrons by using Lithium and oxygen attached to the metal - or an oxygen subgrid in crystal structure.
"In batteries we have metal oxides, that's when a metal bonds with oxygen. In that we put Lithium, which we then build up. That's the principal of how Lithium-ion batteries work. Until now it was generally accepted that we could only use metal-based electrons to store energy, but we have now proven that it can also be efficiently done with oxygen-based electrons," said Dominko. "It's as if a farmer would discover a way for his corn to grow in two layers, both in the ground and above it," is how Dominko illustrated the added value of their discovery.